It works as a greenhouse gas and helps in maintaining the temperature of the earth. Natural sources of carbon dioxide include forest fires, volcanic eruptions, carbonated rocks, etc. It is also the main product of respiration in humans and several other organisms. A lot of you might be wondering one thing, is CO2 heavier than air? In this article, I’ll answer you. So, Is CO2 heavier than air? Yes, CO2 (Carbon Dioxide) is heavier than air. One molecule of carbon dioxide is composed of two oxygen atoms and one carbon atom. Combining the atomic mass of all these atoms, the molecular mass of CO2 is 44 while the average molecular mass of air is around 29. Further, the combined density of air is 1.22 Kg/m3 while that of carbon dioxide is 1.61 Kg/m3. Hence, carbon dioxide is quite heavy in comparison to air. Let’s have a close look at the detailed concept of the CO2 molecule why it is heavier and what affects its density and weight.
Why is CO2 Heavier than Air?
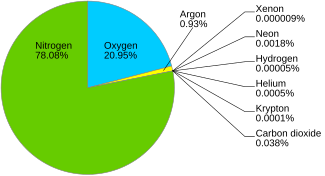
Air is composed of various gases. The main constituents of air are nitrogen and oxygen which constitute 78.08% and 20.95% of the air, respectively. All the other gases viz. carbon dioxide, methane, argon, etc. constitute less than 1% of the air. Check out the article Is Air a homogeneous mixture?
Carbon dioxide constitutes around 0.04% of the air. It is composed of two atoms of oxygen and one atom of carbon. The atomic mass of carbon is 12 while the atomic mass of oxygen is 16. Hence, the molecular mass of CO2 is: 12 + 2 X 16 = 44 On the other hand, the air is a mixture of different gases mostly Nitrogen (almost 80%) and Oxygen (almost 20%). The atomic mass of nitrogen is 14 while the atomic mass of oxygen is 16. Calculating the average molecular mass of air based on the percentage of nitrogen and oxygen:
80% N2 + 20% O2 = (80/100) N2 + (20/100) O2
Adding the values of atomic mass of nitrogen and oxygen
0.8 (2 X 14) + 0.2 (2 X 16) = 0.8 X 28 + 0.2 X 32
= 22.4 + 6.4 = 28.8 Hence, the average molecular mass of air is less than carbon dioxide. Also, the combined density of carbon dioxide is 1.61 Kg/m3 while that of air is 1.22 Kg/m3. This confirms that CO2 is heavier than air.
Does CO2 Sink or Rise in the Air?
Pure carbon dioxide is denser than air and sinks in the air when both are at the same temperature and pressure. However, most of the carbon dioxide released in the atmosphere is a result of natural processes such as volcanic eruptions, or burning of fossil fuels, etc. These processes are exothermic and hence, the carbon dioxide released also attains high temperature due to which its density decreases, and it rises high in the atmosphere. Here, you must understand that air is not stratified into various gases with the densest and heaviest gas at the bottom, while the least dense and lightest gas at the top. If this was the case you would not be able to breathe oxygen. Actually, air exists in the form of a homogeneous mixture of gases owing to the various physical forces of the atmosphere acting on it and also due to the energy of different gas molecules. The regular movement of the collision of molecules amongst themselves does not let the heavier molecules sink or the lighter ones rise high. Hence, stratification of different gases does not occur in the air.
Although, if pure concentrated carbon dioxide is present in a completely undisturbed environment, with stagnant molecules, it will definitely sink in the air. However, it is an ideal condition that does not occur in nature. For more detailed information about bonding in CO2, refer to CO2 Lewis Structure, Geometry, Hybridization.
Is CO2 Heavier than Oxygen?
Yes, when compared at the same temperature and pressure pure carbon dioxide is heavier than pure oxygen. The molecular mass of Oxygen (O2) is 32 while that of Carbon dioxide (CO2) is 44. Also, the density of oxygen is 1.36 while that of carbon dioxide is 1.62. Therefore, carbon dioxide is heavier and denser than oxygen. This can be seen in real life in the bottle of wine. You must have read that wine is made by fermenting different liquids in the presence of oxygen and carbon dioxide is formed during the process of wine formation. Hence, when wine bottles are packed some amount of carbon dioxide released by the wine forms a layer above it not allowing the atmospheric oxygen packed along to interact with the wine.
This stratification of the two gases helps in preserving wine for a long duration otherwise the oxygen may react with the wine spoiling its taste and flavor.
How does CO2 get into the atmosphere if it is heavier than air?
As explained in the previous section stratification of gases does not occur in the air. It is due to the fact that the gas molecules remain in continuous motion due to which they keep colliding and mixing with each other. Owing to regular collisions and movements the gas molecules change their trajectories so fast that it becomes almost impossible for the gravitation forces to act on the gas molecules in the natural environment. Therefore, as soon as CO2 is released in the atmosphere as a product of any process it gets intermixed with various gases in the atmosphere after which it is not possible for the carbon dioxide molecules to settle down in the atmosphere. This is also known as the diffusion of gases.
Smelling the cigarette smoke while standing away from the smoker, being able to recognize someone based on their fragrance from distance, or feeling hungry by smelling tasty food while passing by a restaurant are examples of diffusion.
The Density of CO2 Vs Air
The density of different gases present in the air is given in the table below:
When is CO2 Lighter than Air?
You would have conducted the candle experiment in school in which three candles of different sizes are placed in ascending order height and lit. Thereafter, the candles are covered with a glass container and it is observed which candle will blow last. What did you predict? Most of you would say the shortest candle will blow first as CO2 is heavier than air, but NO. Here, the shortest candle blows out last. Why? We have already discussed in the previous sections that carbon dioxide is heavier than air at room temperature. However, the density of gas significantly depends upon its temperature and pressure. The density of any gas is inversely proportional to temperature i.e. density decreases as the temperature increases. Also, density is directly proportional to pressure i.e. density of any gas increases as the pressure increases. In the candle experiment, the hot carbon dioxide coming out of the burning candles is lighter than the cold air surrounding the candles and forms the upper layer. Therefore, the shortest candles blow out last after all the air of the glass container is consumed.
When the temperature reaches around 140 °C at 1 atm pressure, the density of carbon dioxide is similar to that of air. Similarly, as the pressure reaches around 80 atm at room temperature the CO2 density is almost equal to air. Question: Is CO2 heavier than water? Answer: To answer this question we will have to calculate the molecular weight of water. Water contains two hydrogen atoms and one oxygen atom. The atomic weight of hydrogen is 1 while the atomic weight of oxygen is 16. Therefore, counting the molecular weight of water: H2O = 2 (1) + 16 = 18 The molecular weight of carbon dioxide, as calculated in the earlier sections, is 44 indicating that CO2 is heavier than water. Also, in the gas phase, the density of water vapors is around 0.76 Kg/m3 while that of carbon dioxide is around 1.61 in Kg/m3 validating that CO2 is heavier as well as denser than water. Question: Is CO2 denser as a solid or liquid? Answer: Specific amount of temperature and pressure is required to liquefy or solidify carbon dioxide. Theoretically speaking, CO2 freezes at around 1 atm pressure and −78.5 °C temperature while it transforms from gaseous to the liquid phase at around 56 atm pressure and 20 °C temperature. Also, the density of dry ice i.e. solid carbon dioxide is given as 1562 Kg/m3 while for liquid CO2 density is 770 Kg/m3 indicating that solid carbon dioxide is denser than its liquid form. Question: Is CO2 heavier than nitrogen? Answer: The atomic mass of nitrogen is 14. However, it exists in the atmosphere as N2 molecules, thus, the molecular mass of nitrogen is 28 while the molecular mass of carbon dioxide is 44. Hence, CO2 is heavier than N2. Also, the density of nitrogen is 1.19 in Kg/m3 while that of carbon dioxide is 1.61 in Kg/m3 further indicating that CO2 is heavier and denser than N2. Question: Which is the heaviest gas? Answer: As mentioned in the table given earlier the density of radon is 9.40 in Kg/m3 and its atomic mass is 222 amu. Hence, radon is the heaviest gas. Check out the below an interesting fun video indicating CO2 as heavier than air and sinking down into the empty bottle.
Conclusion
Carbon dioxide is heavier than air as the molecular mass of CO2 is 44 while the average molecular mass of air is around 29. Also, the density of carbon dioxide is 1.61 while the combined density of air is 1.22. Under ideal conditions, CO2 should sink into the air. However, in the atmosphere, a homogeneous mixture of gases form air and due to continuous movement and collision of gas molecules, stratification does not occur. The density of carbon dioxide and the air is almost equal at room temperature when CO2 is placed at 80 atm pressures. Also, carbon dioxide is lighter than air at 140 °C temperature and 1 atm pressure. Carbon dioxide is heavier than most other gases present in the atmosphere viz. oxygen, nitrogen, methane, water vapors, etc. However, argon is known to be the heaviest gas. Happy Reading!! Related Topic: Is CO2 Ionic or Covalent? Is CO2 Organic or Inorganic? Is CO2 Polar or Nonpolar?